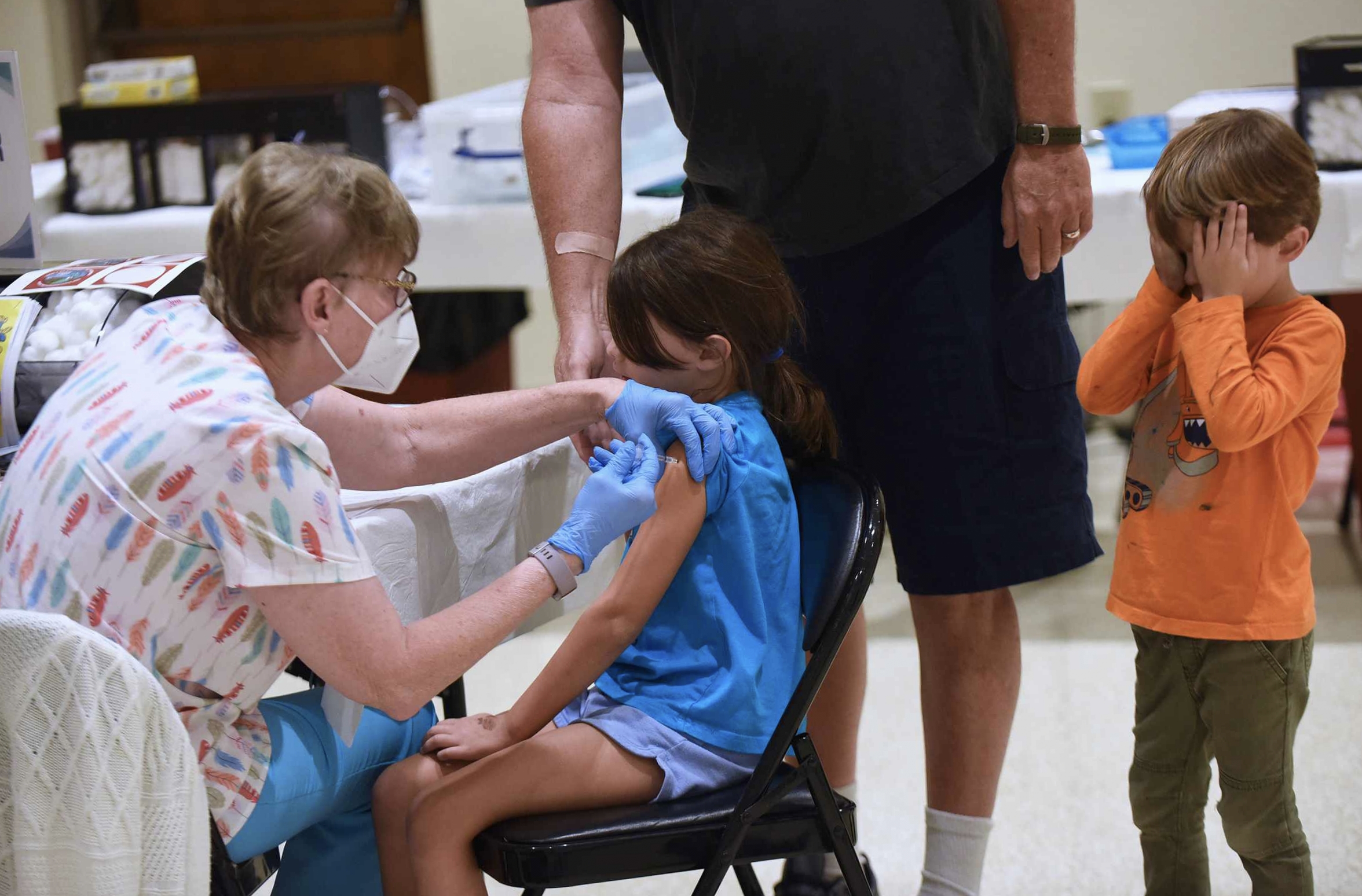
Ottawa: Health Canada has approved Pfizer-BioNTech COVID-19 vaccine, making it the second vaccine option for children six months to four years of age on Friday.
With this, now all children and youth aged 6 months to 17 years can receive a COVID-19 vaccine. Only mRNA vaccines are approved for use in children and youth.
Health Canada in a statement said it has approved COVID-19 vaccines for children and youth aged 6 months and older. We are closely monitoring the rollout of the pediatric vaccine programs in Canada and around the world. It’s very important that we all support children and their caregivers in making informed decisions about COVID-19 vaccination. This means respecting their choices and pace of decision making.
Earlier in July this year, Canada had approved Moderna Spikevax COVID-19 vaccine in children 6 months to 5 years of age.
According to federal data, there are about 1.7 million Canadians under the age of five and over 47, 000 kids have received at least one dose of the vaccine till August 14.
Pfizer-BioNTech officials said that its vaccine is 95% effective in protecting trial participants from COVID-19 for those 16 years and older and effectiveness data supporting the authorization in children ages 6 months to under 5 years are based on a comparison of immune responses in this age group to individuals ages 16 to 25 years.
The data determined that the immune response to the vaccine for both age groups was comparable to the immune response of the older participants.
Pfizer-BioNTech officials said that the dosing for 6 months to less than 5 years is a 3-dose primary series of 3 micrograms each of the vaccine. The initial 2 doses are administered 21 days apart followed by a third dose administered at least 8 weeks after the second dose.