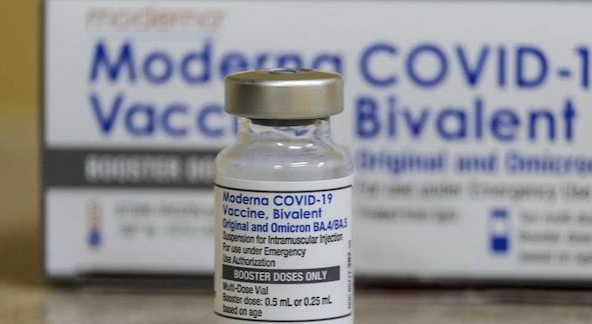
Ottawa: Health Canada today authorized an adapted version of the Moderna Spikevax COVID-19 vaccine that targets the Omicron BA.4/BA.5 subvariants. It is authorized for use as a booster dose in individuals 18 years of age or older.
After a thorough and independent scientific review of the evidence, Health Canada has determined that the bivalent Moderna Spikevax booster is safe and effective. Clinical trial results showed that a booster dose of the bivalent Moderna Spikevax vaccine triggers a strong immune response against both Omicron (BA.4/BA.5) and the original SARS-CoV-2 virus strains.
This adapted vaccine has a similar safety profile to the previously approved Moderna Spikevax boosters, with the same mild adverse reactions that resolved quickly, officials said.
Vaccination continues to be one of the most effective ways to protect families, communities and ourselves against COVID-19. Evidence indicates that the vaccines used in Canada are very effective at preventing severe illness, hospitalization and death from COVID-19. Keeping COVID-19 vaccinations up-to-date, including getting booster doses as recommended, will help protect an individual against serious illness and other complications of COVID-19 infection, officials said.